Jawrani MedTECH Consulting
We Provide Medical Device Research, Design, Engineering, Development & Regulatory Services
Introduction
Jawrani MedTECH Consulting was formed in May of 2019 with a goal of providing research, design, development, engineering and regulatory services to medical device companies to help them launch novel and innovative products aimed at improving the health and lifestyle of their patients.
Services
We provide a wide array of customized services in our core areas of expertise to fit each client’s individual requirements. Each project is discussed in detail to set the expectations for project timelines and deliverables. A clear and open channel of communication is the key to success for any collaborative work, and our business is open around the clock to address the needs of our clients. Our firm specializes in the following areas of expertise:
Research
Experience conducting scientific and clinical research for publication in peer reviewed clinical and scientific journals. Current portfolio of over ten co-authored journal articles.
Design
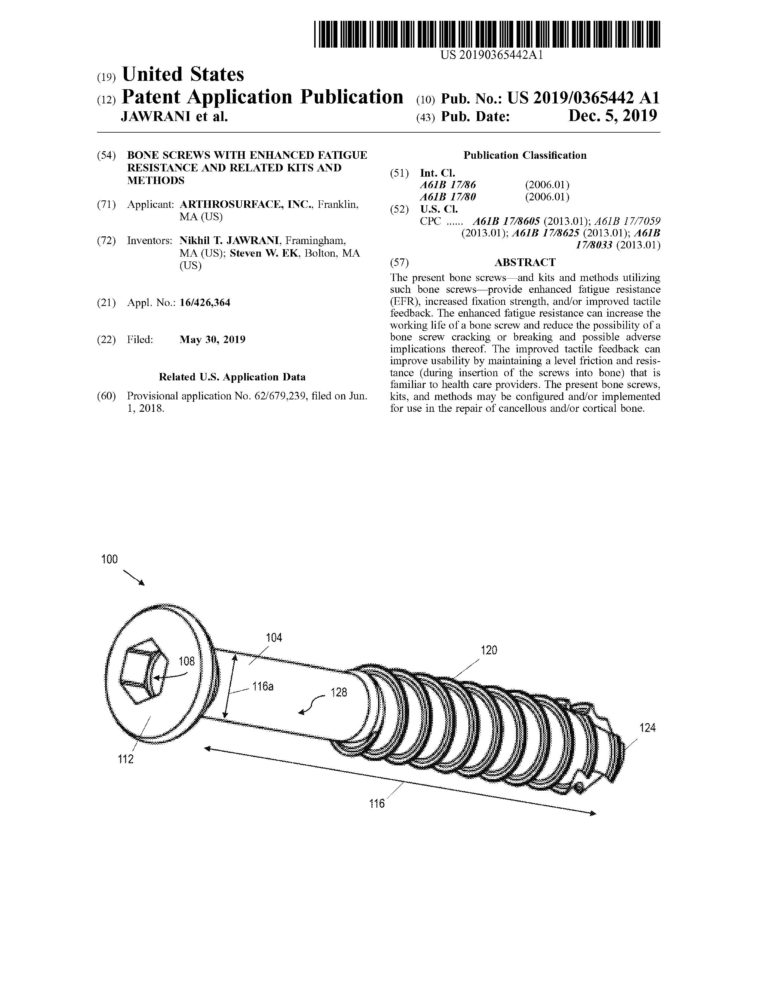
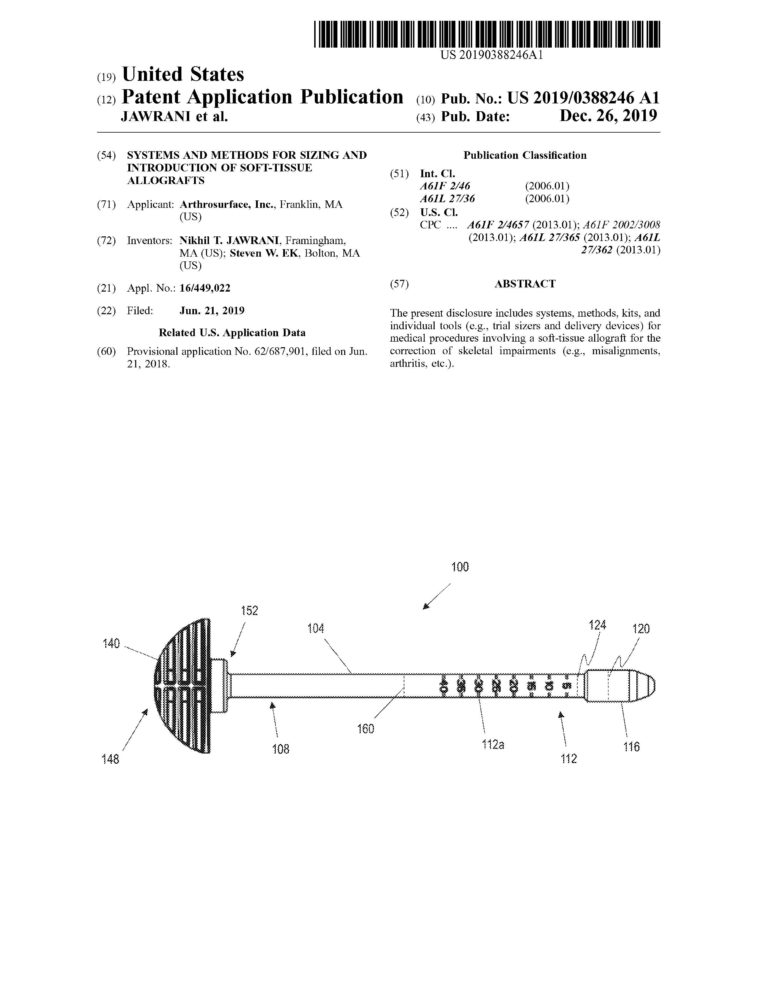
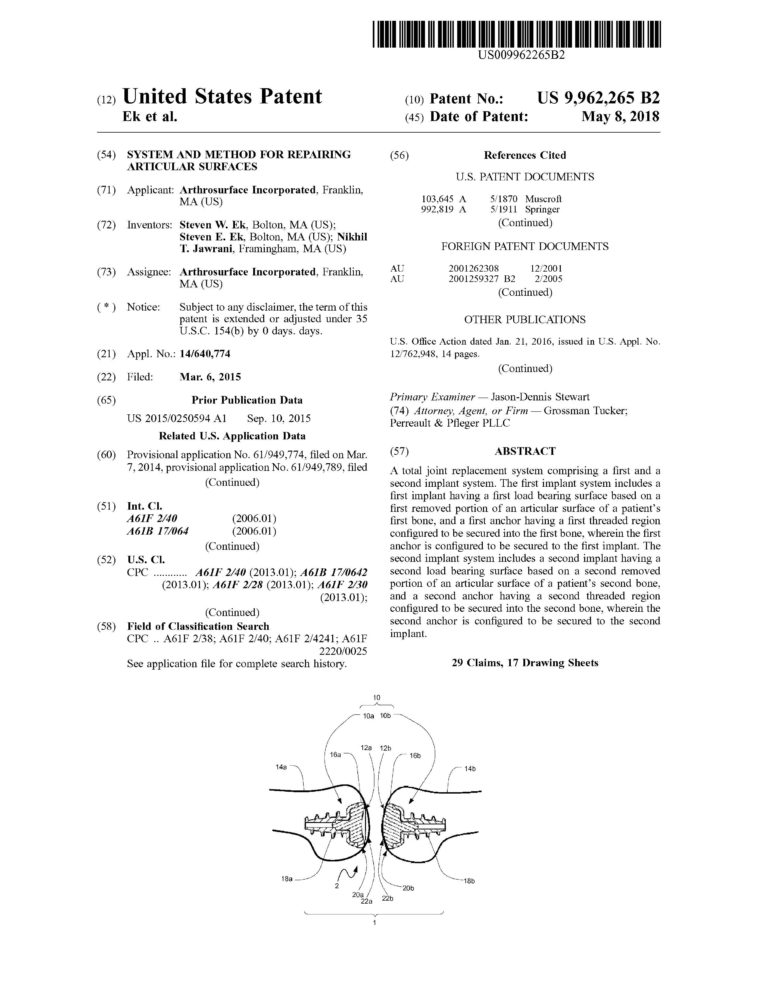
Proficiency in CREO (previously Pro-Engineer) Parametric and Simulation CAD software. Over ten years of experience designing and drafting medical devices that are patented.
Development
Management of complete medical device product life cycle. Experience in all design phases from concept to feasibility to qualification to commercialization.
Engineering
Application of empirical and theoretical engineering principles to solve complex problems for analysis and testing of medical device safety and performance characteristics.
Clinical Evaluation
Authoring and publishing clinical evaluation reports in accordance with requirements of the MDR 2017/745 (previously MEDDEV 2.7.1 rev 4) including detailed analysis of published literature.
Regulatory
Experience with numerous FDA 510(k) filings as well as technical file and design dossier submissions for CE approvals. Thorough understanding of testing requirement specifically for orthopaedic medical devices.
Quality
Compliance strategies for application of FDA's Quality System Regulations and ISO 13485 Quality Management Systems for ensuring safety and effectiveness of medical devices.
Documentation
Project management for timely and accurate documentation of design control documentation such as design history files, technical files and design dossiers.
Consultant

Nikhil T. Jawrani, M.S. Biomedical Engineering
Partner & Chief of R&D
Nikhil holds a Master of Science degree in Biomedical Engineering from Syracuse University in New York. He has more than 12 years of experience working for medical devices companies in the USA where he researched and developed innovative orthopaedic medical devices for the healthcare industry. He is a published author in peer reviewed scientific journals and is a technology inventor listed on multiple patents. He leads client projects as the technical expert for the company's core areas of service.
Experience
2019 - Present
Partner
@Jawrani MedTECH Consulting
2011 - 2019
Project Engineer
@Arthrosurface
2010 - 2011
Research Engineer
@GraMedica
Education
2011-2012
Medical Product Development
@UC Irvine Extension
2007-2010
M.S. in Biomedical Engineering
@Syracuse University
2003-2007
B.E. in Biomedical Engineering
@University of Mumbai
Testimonials
Below are some of our client testimonials:
Steven W. Ek; VP of Research & Development at Anika, Inc.
“I have worked with Nik for many years on Orthopedic implant and instrument product development projects. He has a remarkable ability to work with surgeon customers, extract the critical elements of the product design, and manage the project through its various phases of development. In addition, he can fully document the project and even prepare and submit regulatory filings. He has a very broad base of talents, which is exceptionally valuable and rare. He is a huge asset.”
Michael E. Graham; Founder & CEO of GraMedica
“It is my great pleasure to recommend Jawrani MedTECH Consulting to any company looking for a proactive team. Mr. Jawrani is very experienced and goal oriented leader who will accomplish the desired task. He is a “let’s roll up our sleeves and get to work” individual. If any problems arise, he will find solutions to overcome those challenges. I highly recommend Nikhil and the rest of his team.”
Contact Us
Consulting:
Get in touch with us at nikhil@jawrani.com for all consulting related inquiries.
Jobs:
We are always looking to hire Biomedical Engineers to join our team. Whether you are a fresher or an experienced individual, get in touch with us at careers@jawrani.com to explore new opportunities in the exciting space of medical devices.